NEWSSan Jose, CA – September 4, 2020 (PRESS RELEASE) Biomed Industries, Inc. (Headquarters: San Jose, California, USA) announced today the initiation of a Phase 3, randomized, double blind, placebo-controlled study to evaluate the efficacy and safety of oral polio vaccine and NA-831™ for prophylaxis and treatment of early onset of Covid-19. The clinical protocol has been published on the NIH U.S. National Library of Medicine- Clinical Trials.gov - NCT04540185 Both poliovirus and coronavirus are positive-strand RNA viruses and they may induce and be affected by common innate immunity mechanisms. Recent reports indicate that COVID-19 may result in suppressed innate immune responses. Early clinical studies showed that oral polio vaccine (OPV) protected against poliomyelitis, in addition to reducing the number of other viruses that could be isolated from immunized children, compared with placebo recipients. Large-scale clinical studies of OPV for non-specific prevention of diseases were carried out in the 1960s and 1970s. These involved more than 60,000 individuals and showed that OPV was effective against influenza virus infection, reducing morbidity 3.8-fold on average. SARS-CoV-2 viruses (Covid-19) can directly invade the nervous system of patients, instead of injuring the nervous system through the immune response. It was observed that patients surviving Covid-19 are at high risk for subsequent development of neurological disease and in particular Alzheimer's disease. NA-831™ (Traneurocin™) is a new neuroprotective and neurogenesis drug that has been demonstrated its safety and efficacy in Phase 2A for the treatment of early onset of Alzheimer's disease. NA-831 in oral formulation is well tolerated and has no adverse effects. The Phase 3 study is expected to enroll up to 3,600 participants and will be started by end of November 2020 to the first quarter 2021. NA-831™ NA-831™ (Traneurocin™)is a small drug molecule that exhibits neuroprotection, i.e. can prevent or slow disease progression by halting or at least slowing the loss of neurons. The drug also exhibits neurogenesis, and cognitive protective properties across a range of disease models. Neurogenesis is the process by which new neurons are generated from neural stem cells in the adult. Neurogenesis is reported to play a role in learning, memory and cognitive functions. NA-831 been shown to be safe and well tolerated in healthy volunteers in Phase 1 study, and safety and efficacy in a Phase 2A with patients with early onset of Alzheimer's disease. NA-831 is in Phase 2/3 clinical trials with FDA approved antiviral drugs, Atazanavir and Dexamethasone for the treatment of Covid-19. Oral Polio Vaccine The oral poliovirus vaccine (OPV) developed by Albert Sabin in the 1950s have many advantages for use in polio eradication. Two vaccines, live attenuated oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV), are used to protect against polio. In most countries, a combination of bivalent OPV (type 1 and type 3) and IPV is used. OPV is cheaper than IPV. It replicates in the recipient’s gut, eliciting superior primary intestinal immunity, compared with IPV, and thus is more effective to prevent transmission of wild viruses. OPV confers contact immunity through indirect immunization of unvaccinated persons from viruses shed by vaccinees and it is administered in oral drops, which makes it easier to administer, store, and transport than IPV injections. NeuroActiva Safe Harbor This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements about results from the Phase II study of NA-831; the potential clinical effects of NA-831; the potential benefits, safety, and efficacy of NA-831; the clinical development program for NA-831; risks and uncertainties associated with drug development and commercialization; the treatment of Alzheimer’s disease; NeuroActiva’s strategy and plans; and the potential of NeuroActiva’s commercial business and pipeline programs, including NA-831. These forward-looking statements may be accompanied by words such as “aim,” “anticipate,” “believe,” “could,” “estimate,” “except,” “forecast,” “intend,” “may,” “plan,” “potential,” “possible,” “will,” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements or the scientific data presented. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including, without limitation: the risk that clinical trials may not fully enroll or enrollment will take longer than expected; unexpected concerns may arise from additional data, analysis, or results obtained during clinical trials; regulatory authorities may require additional information or further studies, or may fail or refuse to approve or may delay approval of NeuroActiva’s drug candidates, including NA-831 ; the occurrence of adverse safety events; NeuroActiva may encounter other unexpected hurdles; uncertainty of success in the development and potential commercialization of NA-831, which may be impacted by, among other things, unexpected concerns that may arise from additional data or analysis, the occurrence of adverse safety events, failure to obtain regulatory approvals in certain jurisdictions, failure to protect and enforce NeuroActiva ’s data, intellectual property, and other proprietary rights and uncertainties relating to intellectual property claims and challenges. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from NeuroActiva’s expectations in any forward-looking statement. These statements are based on NeuroActiva’s current beliefs and expectations and speak only as of the date of this press release. NeuroActiva does not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
MEDIA CONTACTS NeuroActiva™ Inc. announced the initiation of clinical study of NA-831 and Remdesivir (GS-5734) in nanoparticle inhalation formulation for the treatment of early onset of Covid-19.San Jose, CA – July 21, 2020 (PRESS RELEASE) The clinical protocol has been published on the NIH U.S. National Library of Medicine- Clinical Trials.gov - NCT04480333. It has been discovered that SARS-CoV-2 viruses (Covid-19) can directly invade the nervous system of patients, instead of injuring the nervous system through the immune response. Neurotropism is one common feature of Covid-19. Increasing evidence suggests that infection with SARS-CoV-2 causes neurological deficits in a substantial proportion of affected patients. It was observed that patients surviving COVID-19 are at high risk for subsequent development of neurological disease and in particular Alzheimer's disease. NA-831™ is a new neuroprotective and neurogenesis drug that has been demonstrated its promising safety and efficacy in Phase 2A for the treatment of early onset of Alzheimer's disease. NA-831 in oral formulation is well tolerated NA-831 with no adverse effects. NA-831 in oral formulation exhibits predictable pharmacokinetics including dose-dependent exposure linearity and low variability. Based on animal studies, NA-831 can provide effective interventions during the severe acute respiratory syndrome, and provide appropriate rehabilitation measures afterwards. Remdesivir™ (GS-5734- manufactured by Gilead Sciences, Inc.) intravenous formulation has been approved by the FDA under the emergency use authorization for potential treatment of severe cases of Covid-19. It was found the upper respiratory tract is the most prevalent site of SARS-CoV-2 infection early in disease. Delivering drugs directly to the primary site of infection with a nebulizer, inhaled nanoparticle formulation may enable more targeted and accessible administration in non-hospitalized patients and potentially lower systemic exposure to the drug. The study is designed to evaluate the safety, tolerability and pharmacokinetics of a new nanoparticle formulation of Remdesivir (GS-5734) and combination therapy with NA-831 in healthy volunteers. The study is expected to begin enrolling on September 15, 2020 and complete by March 31 2021. In addition, NeuroActiva has initiated a Phase 2/3 clinical study to evaluate the safety and efficacy of NA-831 alone, and a combination therapy comprises NA-831 with an anti-viral drug Atazanavir, NA-831 with an anti-inflammatory drug Dexamethasone. (clinicaltrials.gov- NCT04452565) NA-831™ is a small drug molecule that exhibits neuroprotection, i.e. can prevent or slow disease progression by halting or at least slowing the loss of neurons. The drug also exhibits neurogenesis, and cognitive protective properties across a range of disease models. Neurogenesis is the process by which new neurons are generated from neural stem cells in the adult. Neurogenesis is reported to play a role in learning, memory and cognitive functions. NA-831™ has been shown to be safe and well tolerated in healthy volunteers in Phase 1 study, and safety and efficacy in a Phase 2A with patients with early onset of Alzheimer's disease. Remdesivir™ ( Gilead Science, Inc.) has been issued an Emergency Use Authorization by the US Food & Drug Administration (FDA) for the treatment of hospitalized patients with severe COVID-19. Remdesivir™ is an investigational antiviral drug that is being studied in multiple ongoing international clinical trials, and the safety and efficacy of remdesivir for the treatment of COVID-19 are not yet established. Remdesivir™ has not been approved by the US FDA for any use. For information about the authorized use of remdesivir and mandatory requirements of the Emergency Use Authorization in the US, please review the Fact Sheet for Healthcare Providers and FDA Letter of Authorization available at www.gilead.com/remdesivir. This press release discusses investigational uses of agents in development. All trademarks used or mentioned in this release are protected by law. NeuroActiva Safe HarborThis press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements about results from the Phase II study of NA-831; the potential clinical effects of NA-831; the potential benefits, safety, and efficacy of NA-831; the clinical development program for NA-831; risks and uncertainties associated with drug development and commercialization; the treatment of Alzheimer’s disease; NeuroActiva’s strategy and plans; and the potential of NeuroActiva’s commercial business and pipeline programs, including NA-831. These forward-looking statements may be accompanied by words such as “aim,” “anticipate,” “believe,” “could,” “estimate,” “except,” “forecast,” “intend,” “may,” “plan,” “potential,” “possible,” “will,” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements or the scientific data presented. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including, without limitation: the risk that clinical trials may not fully enroll or enrollment will take longer than expected; unexpected concerns may arise from additional data, analysis, or results obtained during clinical trials; regulatory authorities may require additional information or further studies, or may fail or refuse to approve or may delay approval of NeuroActiva’s drug candidates, including NA-831 ; the occurrence of adverse safety events; NeuroActiva may encounter other unexpected hurdles; uncertainty of success in the development and potential commercialization of NA-831, which may be impacted by, among other things, unexpected concerns that may arise from additional data or analysis, the occurrence of adverse safety events, failure to obtain regulatory approvals in certain jurisdictions, failure to protect and enforce NeuroActiva ’s data, intellectual property, and other proprietary rights and uncertainties relating to intellectual property claims and challenges. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from NeuroActiva’s expectations in any forward-looking statement. These statements are based on NeuroActiva’s current beliefs and expectations and speak only as of the date of this press release. NeuroActiva does not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
MEDIA CONTACTS NeuroActiva™ Inc. presented updated results of Phase 2A Clinical Trial and Phase 3 Treatment and Prevention Study Protocols of NA-831™ at the Clinical Trials on Alzheimer’s Disease Conference, December 4-7, 2019 in San Diego, CaliforniaSan Jose, CA – December 6, 2019 (PRESS RELEASE) NeuroActiva™ Inc. (Headquarters: Sunnyvale, California, USA) presented updated results of the Phase 2A clinical study of the investigational oral NA-831 at the 12th Clinical Trials on Alzheimer’s Disease (CTAD) on December 4-7, 2019 in San Diego, California, United States. The paper titled “A Randomized Double-Blind Placebo-Controlled Phase 2A Clinical Trial of NA-831™ in Patients with MCI and Mild and Moderate Alzheimer’s Disease” was accepted as a late breaking oral communication CTAD 2019 on Friday December 6, 2019. In addition, the company gave two poster presentations on the Phase 3 Prevention and Treatment Study Protocol at the CTAD 2019. A Phase 3- Efficacy and Safety Study Protocol of Traneurocin™ (NA-831™) in Participants Who Are Asymptomatic at Risk for Developing Alzheimer's Dementia (PREVENTION) A 48-Week Phase 3 Clinical Trial Method to Evaluate the Efficacy and Safety of NA-831™ in Subjects With Early Alzheimer's Disease (TREATMENT) Alzheimer’s Disease is a progressive and irreversible neurodegenerative disorder that affects millions of people worldwide. Today, more than 5.8 million people in the United States and 45 million people worldwide live with Alzheimer’s. There is currently no cure, no prevention and no treatment of Alzheimer’s disease. The study was a randomized, double-blind, placebo-controlled parallel-group Phase 2A clinical study of NA-831, conducted in patients with mild cognitive impairment (MCI), and patients with mild and moderate Alzheimer’s disease over 24 weeks, with a follow-up for an additional 24 weeks. In addition to the primary safety objective, the study assessed the efficacy in terms of clinical symptoms, which were exploratory objectives in this study. Mild cognitive impairment (“MCI”) is defined as the “symptomatic pre-dementia stage” on the continuum of cognitive decline leading to Alzheimer’s disease. In an early stage of Alzheimer's disease (“mild Alzheimer’s) a person may function independently and can still drive, work and be part of social activities. Moderate Alzheimer's is typically the longest stage and can last for many years. During the moderate stage of Alzheimer’s, the dementia symptoms are more pronounced. As the disease progresses, the person with Alzheimer's will require a greater level of care. NA-831™is a small drug molecule that exhibits neuroprotection, i.e. can prevent or slow disease progression by halting or at least slowing the loss of neurons. The drug also exhibits neurogenesis, and cognitive protective properties across a range of disease models. Neurogenesis is the process by which new neurons are generated from neural stem cells in the adult. Neurogenesis is reported to play a role in learning, memory and cognitive functions. NA-831™ has been shown to be safe and well tolerated in healthy volunteers. The results suggest that NA-831 could slow decline in cognitive function of patients with MCI due to Alzheimer’s disease, or mild to moderate dementia due to Alzheimer’s disease. NA-831 was well-tolerated at 30 mg per day administered orally. There were no serious adverse events. This press release discusses investigational uses of agents in development.
NeuroActiva Safe Harbor |
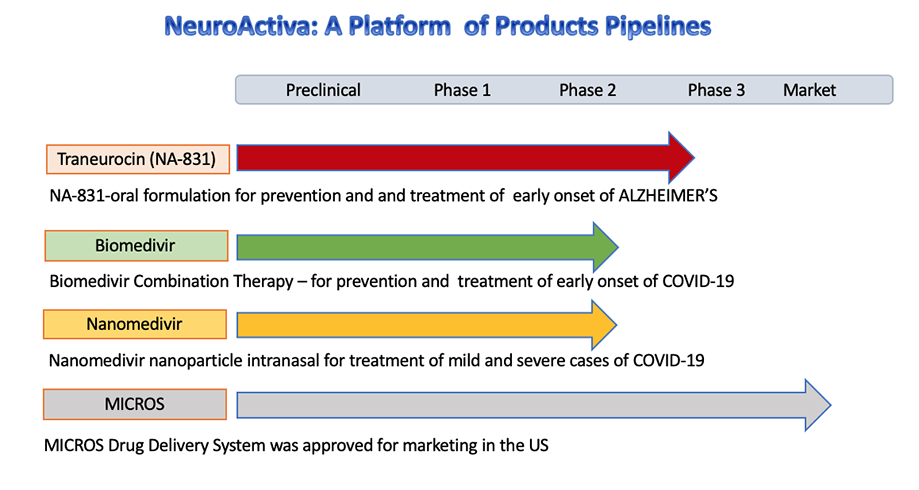
Prophylaxis and treatment for Alzheimer's disease and Covid-19:
-
Covid-19 Neuro-invasion
SARS-CoV-2 can directly invade the CNS. NeuroActiva has developed neuroprotective interventions during the severe acute respiratory syndrome, and provide appropriate rehabilitation measures afterwards.
-
NA-831 (Traneurocin)
(NA-831™)(Traneurocin™) is a novel neuroprotective and neurogenesis drug for treatment of Alzheimer's disease which will be in Phase 3 in 2020-2021
-
NA-831™ Drug Therapy
We have developed drug combination therapy known as Biomedivr™, Neuromedivir™, Dexaneurosone™ Neurosivir™ for the prophylaxis and treatment of Covid-19
-
MICROS™ Infusion System
The MICROS is a FDA approved controlled release infusion system for intravenous administration of injectable drugs, including NA-704 for treatment of neurological diseases.