A PLATFORM OF INNOVATIVE PRODUCTS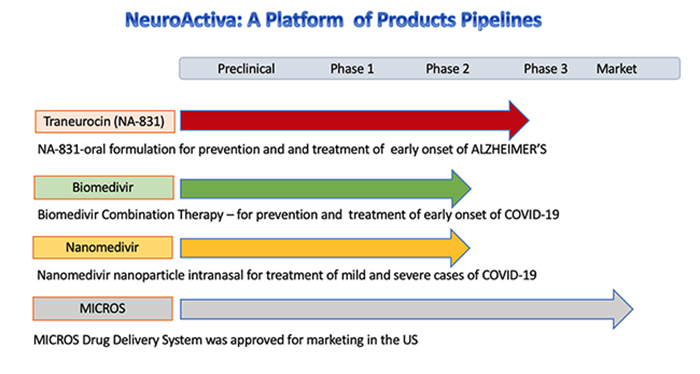
NeuroActiva, Inc. is a wholly owned subsidiary of Biomed Industries, Inc. which is also parent company of Biomed Pharmaceuticals, Inc, Biomed AI and BiopharmaRX. EXPERIENCED DRUG DEVELOPER Biomed Pharmaceuticals, Inc. was a pioneer in developing new anti-malarial and antiviral drugs dated back in 1990s. During that time, there were more 100 million cases of malaria and 1 million people mostly children died in Africa and Asia.The company developed a new drug called Artemether, which has been demonstrated as an effective drug to treat Plasmodium falciparum malaria, that are resistant to chloroquine, which was the standard treatment for malaria at the time. Artemether has been approved as the last defense drug against chloroquine resistant Plasmodium falciparum malaria in more than 80 countries, include the FDA in the USA. Artemether/Lumefantrine is presently distributed worldwide by Novartis International AG. With more than 25 year experience in drug development, Biomed has focused its resources to developing new drugs for the prevention and treatment of Covid-19. COVID-19 According to World Health Organization, 14% of patients with severe COVID-19 require hospitalization and supplemental oxygen, and 5% will require treatment in intensive care units. More than 80% of the patients in early onset of Covid-19 can be treated at home or in alternative care settings. Biomed focuses on the developing new drugs for Covid-19 that can be used to treat mild and moderate cases for patients at home and alternative care settings. Evidence shows that coronaviruses are not always confined to the respiratory tract and that they may also invade the central nervous system inducing neurological diseases. Brainstem has been demonstrated to be heavily infected by SARS‐CoV or MERS‐CoV. CoVs may first invade peripheral nerve terminals, and then gain access to the CNS via a synapse‐connected route. In patients with severe COVID-19, rapid clinical deterioration or worsening could be associated with a neurologic event such as stroke, which would contribute to its high mortality rate. About one third of patients at the time of discharge have evidence of cognitive impairment and motor deficits. Patients surviving COVID-19 are at high risk for subsequent development of neurological disease and in particular Alzheimer’s disease. BIOMED PRODUCTS FOR COVID-19 Our drug treatment strategy is to develop neuroprotective interventions during the severe acute respiratory syndrome, and provide appropriate rehabilitation measures afterwards. Biomed’s strategy is to provide prevention and drug treatment in oral or intranasal formulations to anyone who is at risks (such as healthcare providers, first responders) or patients in early onset of Covid-19 so they can take the medication themselves with the physician prescription. 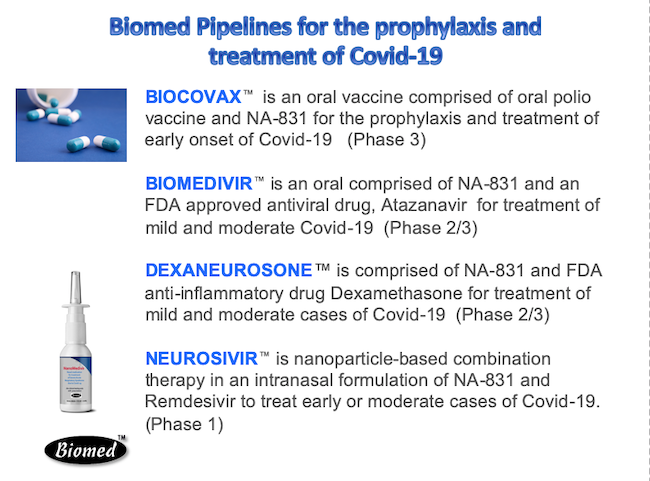 BIOCOVAX™ Biomed has developed Biovaccine™, which is comprised of an oral polio vaccine with our neuroprotective agent called NA-831. The oral polio vaccine has been approved by the FDA and widely used in the US until 2004 when the polio was declared eradicated in the country. BioCovax™ is an oral formulation which can be used as a prophylaxis and treatment of early onset of Covid-19. Biomed plans to launch the Phase 3 trials of BioCovax™ in early autumn 2020. BIOMEDIVIR™ Biomed has developed Biomedivir™, which is comprised of our neuroprotective agent called NA-831 and Atazanavir. Atazanavir was approved by the FDA for prevention and treatment of HIV/AIDS treatment, and is now a generic drug. Biomedivir™ is an oral formulation which can be used as treatment for mild and moderate of Covid-19 infections. Biomed plans to launch the Phase 2/3 trials of Biomedivir™ in early autumn 2020. NANOMEDIVIR™ Biomed has developed Nanomedivir™, which is comprised of NA-831 and Remdesivir, anti-viral drug in nanoparticle-based intranasal formulation. Remdesivir (made by Gilead Sciences has been approved by the FDA for emergency use of severe cases of Covid-19. Biomed plan to launch the Phase 1 clinical trials of Nanomedivir™ in autumn 2020. DEXANEUROSONE™ In addition, Biomed has also developed Dexaneurosone™, which is comprised of NA-831 and Dexamethasone in both oral and intranasal formulation. Dexamethasone is an FDA anti-inflammatory drug has been on the market for some 60 years and is used to treat conditions including arthritis and severe asthma. DDexaneurosone™ could be used to treat early and moderate cases of Covid-19. Biomed plan to launch the Phase 2/3 clinical trials of Dexaneurosone™ in autumn 2020. NOTE: BioCovax, Biomedivir, Nanomedivir and Dexaneurosone are experimental drugs in clinical evaluation, and are not available to patients in the USA, until we receive the FDA approval. Patients should consult with physicians about the drugs. For further information, please contact us by completing Contact an Online Contact Form. Thank you. |
Prophylaxis and treatment for Alzheimer's disease and Covid-19:
-
Covid-19 Neuro-invasion
SARS-CoV-2 can directly invade the CNS. NeuroActiva has developed neuroprotective interventions during the severe acute respiratory syndrome, and provide appropriate rehabilitation measures afterwards.
-
NA-831 (Traneurocin)
(NA-831™)(Traneurocin™) is a novel neuroprotective and neurogenesis drug for treatment of Alzheimer's disease which will be in Phase 3 in 2020-2021
-
NA-831™ Drug Therapy
We have developed drug combination therapy known as Biomedivr™, Neuromedivir™, Dexaneurosone™ Neurosivir™ for the prophylaxis and treatment of Covid-19
-
MICROS™ Infusion System
The MICROS is a FDA approved controlled release infusion system for intravenous administration of injectable drugs, including NA-704 for treatment of neurological diseases.